Home > Press > New hydrogen-storage method discovered
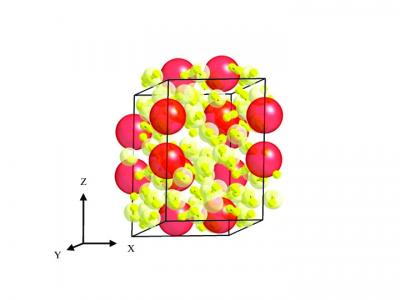 |
| This schematic shows the structure of the new material, Xe(H2)7. Freely rotating hydrogen molecules (red dumbbells) surround xenon atoms (yellow). Credit: Nature Chemistry |
Abstract:
Scientists at the Carnegie Institution have found for the first time that high pressure can be used to make a unique hydrogen-storage material. The discovery paves the way for an entirely new way to approach the hydrogen-storage problem.
New hydrogen-storage method discovered
Washington, DC | Posted on November 24th, 2009The researchers found that the normally unreactive, noble gas xenon combines with molecular hydrogen (H2) under pressure to form a previously unknown solid with unusual bonding chemistry. The experiments are the first time these elements have been combined to form a stable compound. The discovery debuts a new family of materials, which could boost new hydrogen technologies. The paper is published in the November 22, 2009, advanced online publication of Nature Chemistry.
Xenon has some intriguing properties, including its use as an anesthesia, its ability to preserve biological tissues, and its employment in lighting. Xenon is a noble gas, which means that it does not typically react with other elements.
As lead author Maddury Somayazulu, research scientist at Carnegie's Geophysical Laboratory, explained: "Elements change their configuration when placed under pressure, sort of like passengers readjusting themselves as the elevator becomes full. We subjected a series of gas mixtures of xenon in combination with hydrogen to high pressures in a diamond anvil cell. At about 41,000 times the pressure at sea level (1 atmosphere), the atoms became arranged in a lattice structure dominated by hydrogen, but interspersed with layers of loosely bonded xenon pairs. When we increased pressure, like tuning a radio, the distances between the xenon pairs changed-the distances contracted to those observed in dense metallic xenon."
The researchers imaged the compound at varying pressures using X-ray diffraction, infrared and Raman spectroscopy. When they looked at the xenon part of the structure, they realized that the interaction of xenon with the surrounding hydrogen was responsible for the unusual stability and the continuous change in xenon-xenon distances as pressure was adjusted from 41,000 to 255,000 atmospheres.
Why was the compound so stable? "We were taken off guard by both the structure and stability of this material," said Przemek Dera, the lead crystallographer who looked at the changes in electron density at different pressures using single-crystal diffraction. As electron density from the xenon atoms spreads towards the surrounding hydrogen molecules, it seems to stabilize the compound and the xenon pairs.
"Xenon is too heavy and expensive to be practical for use in hydrogen-storage applications," remarked Somayazulu. "But by understanding how it works in this situation, researchers can come up with lighter substitutes."
"It's very exciting to come up with new hydrogen-rich compounds, not just for our interest in simple molecular systems, but because such discoveries can be the foundation for important new technologies," commented Russell Hemley, director of the Geophysical Laboratory and a co-author. "This hydrogen-rich solid represents a new pathway to forming novel hydrogen storage compounds and the new pressure-induced chemistry opens the possibility of synthesizing new energetic materials."
This research was funded by the Department of Energy, Basic Energy Sciences hydrogen storage, and the National Science Foundation, Division of Materials Research.
####
About Carnegie Institution
The Carnegie Institution for Science (www.CIW.edu) has been a pioneering force in basic scientific research since 1902. It is a private, nonprofit organization with six research departments throughout the U.S. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
For more information, please click here
Contacts:
Maddury Somayazulu
202-478-8911
Copyright © Carnegie Institution
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








