Home > Press > Simultaneous Oxidation and Reduction of Arsenic by Zero-Valent Iron Nanoparticles: Understanding the Significance of the Core−Shell Structure
 |
Abstract:
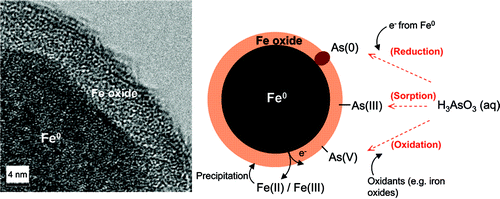
Increasing evidence suggests that nanoscale zerovalent iron (nZVI) is effective for the removal of arsenic from contaminated water, but the immobilization mechanism is unclear. In particular, the existence of As(0) on the nanoparticle surface has been proposed but not substantiated in prior studies. By using high-resolution X-ray photoelectron spectroscopy (HR-XPS), we report clear evidence of As(0) species on nZVI surfaces after reactions with As(III) or As(V) species in solutions. These results prove that reduction to elemental arsenic by nZVI is an important mechanism for arsenic immobilization. Furthermore, reactions of nZVI with As(III) generated As(0), As(III), and As(V) on the nanoparticle surfaces, indicating both reduction and oxidation of As(III) take place with nZVI treatment. The dual redox functions exhibited by nZVI are enabled by its core−shell structure containing a metallic core with a highly reducing characteristic and a thin amorphous iron (oxy)hydroxide layer promoting As(III) coordination and oxidation. Results demonstrated here shed light on the underlying mechanisms of arsenic reactions with nZVI and suggest nZVI as a potential multifaceted agent for arsenic remediation.
Simultaneous Oxidation and Reduction of Arsenic by Zero-Valent Iron Nanoparticles: Understanding the Significance of the Core−Shell Structure
Washington, DC | Posted on August 5th, 2009Arsenic is a well-known carcinogen, and arsenic contaminated groundwater is prevalent around the world, particularly in South Asia. In the United States, groundwater supplies about one-third of the country's drinking water. However, there are many locations in the southwestern states where groundwater contains arsenic concentrations in excess of the World Health Organization (WHO) guidelines and the U.S. Environmental Protection Agency (USEPA) drinking water standard of 10 ìg/L.(1-3) On a worldwide scale, it has been estimated that over 137 million people in more than 70 countries are affected by arsenic poisoning of drinking water.(4)The principal forms of arsenic in natural waters are arsenate [As(V)] and arsenite [As(III)]. Arsenate exists as oxyanions (H2AsO4− or HAsO42−) in a pH range of 2−12, while arsenite remains as neutral undissociated species (H3AsO3) below a pH of 9.2.(5, 6) As(III) oxidation to As(V) by dissolved oxygen alone is kinetically slow.(4, 7) Traditional remediation technologies rely largely on adsorption for removing arsenic in water using materials such as iron oxides, activated alumina, granular activated carbon, and silica with various degrees of success.(9, 10) Recent studies suggest that nanoscale zerovalent iron (nZVI), a highly reactive engineered nanomaterial extensively evaluated for groundwater remediation and hazardous waste treatment, is effective for arsenic removal in water, but the reaction mechanisms of arsenic on nZVI are not well understood. Because of nZVI's strong reducing capabilities toward chlorinated hydrocarbons and numerous inorganic contaminants, it is expected that both arsenic(V) and arsenic(III) are amenable to reduction by nZVI. Prior publications indicate, however, the transformation of As(V) to As(III) is kinetically slow, and no clear evidence for the formation of elemental arsenic [As(0)] has been published so far.(5, 11, 12) On the other hand, several recent studies suggest that, instead of reduction, oxidation of arsenic(III) is the prevalent reaction under oxic conditions through peroxide or radicals generated by Fenton reactions.(8, 13) The majority of these studies rely on solution phase arsenic analysis, and there is a lack of studies investigating arsenic speciation on nZVI surfaces that can provide more direct evidence of the reactions between nZVI and arsenic.
The objective of this work was to examine surface reactions of arsenate and arsenite with nZVI by using high-resolution X-ray photoelectron spectroscopy (HR-XPS), which can provide valuable information about the valence states and abundance of various arsenic species on nanoparticle surfaces. The results are interpreted in light of the fundamental structure of nZVI, and the implications of these findings on arsenic treatment and remediation are discussed.
Materials and Experimental Methods
The iron nanoparticles (nZVI) used in this study were prepared from sodium borohydride reduction of ferric iron as reported previously.(14, 15) The nanoparticles have a mean diameter of 60 nm and a BET-measured surface area of 30 m2/g.(14) Reagent grade sodium arsenate or arsenite was used to make As(III) or As(V) stock solutions. To eliminate dissolved O2 as a potential oxidant in these solutions and to simulate prevalent anoxic conditions encountered in groundwater environments, the arsenic solutions were purged with high-purity N2 for 30 min prior to adding nZVI. HR-XPS analysis was performed on reacted nZVI residues using a Scienta ESCA 300 X-ray photoelectron spectrometer. Detailed experimental procedures and details concerning XPS analysis including spectral deconvolution are available in the Supporting Information.
Conclusion
Simultaneous oxidation and reduction of As(III) and the reduction of As(V) to As(III) and As(0) on nZVI surfaces have been observed. The elusive As(0) species suggested in previous studies was clearly detected. The reactions can be reasonably explained on the basis of the core−shell structure of nZVI, in which the oxide outer layer and metallic core bestow distinctive mechanisms for arsenic removal. The results shown here may help in the understanding of the reaction mechanisms and the stability of arsenic on ZVI surfaces and lead to better design of arsenic sequestration technologies. In particular, in view of the conventional adsorptive methods, which are critically affected by arsenic speciation, concentration, and pH, the multifaceted functionality of nZVI exhibited here may offer a more robust and effective method for arsenic removal.
Read the entire article, here: pubs.acs.org/stoken/campaign/acs/full/10.1021/jp9051837?cookieSet=1
Adapted from materials provided by American Chemical Society.
####
About American Chemical Society
ACS is a congressionally chartered independent membership organization which represents professionals at all degree levels and in all fields of chemistry and sciences that involve chemistry.
For more information, please click here
Contacts:
American Chemical Society
1155 Sixteenth Street, NW
Washington, DC 20036
USA
(800) 227-5558 (US)
(202) 872-4600 (Worldwide)
Media Inquiries
Copyright © American Chemical Society
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








