Home > Press > Rolling out the nanotubes: Synthesis of graphitic nanotubes containing platinum metals achieved through self-assembly techniques
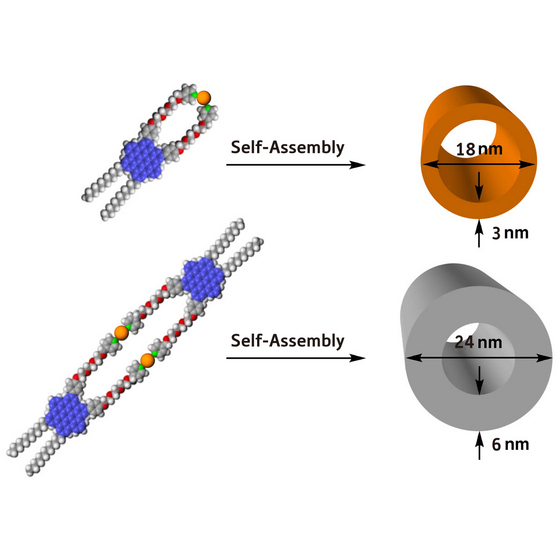 |
| Figure 1: Two examples of nanotubular assemblies fabricated from single hexabenzocoronene amphiphile building blocks (blue/grey/red spheres) and platinum (Pt) metal ions (orange spheres). Credit: Riken |
Abstract:
Nanoscale materials with well-defined shapes, such as one-dimensional hollow tubes, have attracted the interest of scientists seeking to utilize their unique properties. Nanotubes have large inner and outer surface areas that are accessible to many smaller molecules, meaning they have the potential to be developed into new types of sensors and catalysts.
Rolling out the nanotubes: Synthesis of graphitic nanotubes containing platinum metals achieved through self-assembly techniques
Japan | Posted on July 24th, 2009Efficient techniques to synthesize nanotubes, however, are uncommon. Now, Takuzo Aida and Takanori Fukushima of the RIKEN Advanced Science Institute in Wako and colleagues from the Japan Science and Technology Agency have developed a way to controllably self-assemble graphitic molecules and platinum metals into nanotubes with specific dimensions and structural features1.
Aida and his team used a molecule called hexabenzocoronene (HBC) as the base for their new nanotubes. Consisting of thirteen aromatic benzene rings interlocked into a large, flat cyclic structure that resembles graphite, HBC is normally used as a building block for liquid crystalline semiconductors.
In 2004, Aida, Fukushima, and colleagues discovered that by adding long hydrocarbon groups and polar chains called triethylene glycol to HBC, they could make the graphitic molecule into an amphiphile2—a surfactant that can be dissolved in organic solvents. Recrystallizing a solution of the HBC amphiphiles spontaneously produced new graphitic nanotubes.
In their latest work, the researchers incorporated platinum metals into their nanotubes structures. According to Fukushima, transition metals such as platinum can add useful catalytic, electronic, luminescent, and magnetic functionalities to the nanotubes.
In order to attach platinum metals to the nanotubes, the scientists added a molecule known as pyridine, a nitrogen-containing benzene ring, to the ends of the triethylene glycol chains on the HBC amphiphile.
"Pyridine is one of the simplest and most common molecules for binding transition metals," explains Fukushima. "We thought it fit to use such a general binding molecule in our first attempt to functionalize the HBC nanotubes with transition metals."
By heating a solution of the HBC amphiphiles with platinum metal ions, then allowing the mixture to cool to room temperature, the scientists observed spontaneous formation of new metal-ion-coated graphitic nanotubes (Fig. 1). Altering the assembly conditions produced tubular assemblies with different diameters, lengths, and wall widths.
"Our nanotube can serve as a unique one-dimensional nano-scaffold with not only high structural integrity, but also with beneficial electronic properties such as energy and charge transport capabilities," says Fukushima. "We expect that the combination of these two components might lead to unprecedented phenomenon and functions."
Reference
1. Zhang, W., Jin, W., Fukushima, T., Ishii, N. & Aida, T. Metal-ion-coated graphitic nanotubes: controlled self-assembly of a pyridyl-appended gemini-shaped hexabenzocoronene amphiphile. Angewandte Chemie International Edition 121, 4841-4844 (2009).
2. Hill, J. P., Jin, W., Kosaka, A., Fukushima, T., Ichihara, H., Shimomura, T., Ito, K., Hashizume, T., Ishii, N. & Aida, T. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube. Science 304, 1481-1483 (2004).
The corresponding author for this highlight is based at the RIKEN Functional Soft Matter Engineering Team
####
For more information, please click here
Copyright © Riken
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Self Assembly
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
![]() Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








