Home > Press > Skipping Atomic-scale Stones to Study Some Chemistry Basics
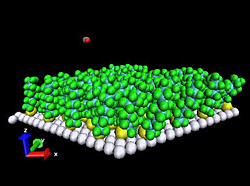 |
| Computer simulation of the JILA gas-liquid scattering experiments uses long molecules tethered to a surface as a useful stand-in for liquids, which are too complex for computer modeling. The speed of rotation of the carbon dioxide molecule after striking the surface is strongly dependant on its orientation, an effect caused by atomic-scale “waves” on the fluid surface. |
Abstract:
Thought experiment: a carbon dioxide molecule—think of a cheerleader's baton—comes slanting in at high speed over a dense liquid, strikes the surface and ricochets. How does it tumble? Fast or slow? Forward, backward or sideways? These are not idle questions because simple events like the tumbling molecule go to the heart of the chemistry and physics of gas-liquid interactions. These cover a broad swath of important chemical processes—including breathing—for which details of the encounter are just coming into view.
Skipping Atomic-scale Stones to Study Some Chemistry Basics
GAITHERSBURG, MD | Posted on August 6th, 2008New experiments reported this week* from JILA in Boulder, Colo., are giving a uniquely detailed look at what happens when gas molecule meets fluid.
Historically, chemistry has been confined to observing the mass behavior of huge numbers of molecules—mix things together, look at the reaction products, infer what happened. Only in the past couple of decades have powerful lasers made it possible to "watch" specific events involving only a few molecules. Today, they can even observe the role played by a molecule's shape, a critical influence in many interactions.
Now, Bradford Perkins, Jr., of the University of Colorado and David Nesbitt of the National Institute of Standards and Technology (NIST) report the first direct observation of the rotational dynamics of a molecule bouncing off a liquid surface.
Perkins and Nesbitt directed a beam of carbon dioxide molecules at a pool of synthetic fluorinated fluid in a vacuum. The molecules that bounced off passed through an infrared laser beam, which switched rapidly between alternate orientations, or polarization states. A sensitive detector measured how much light was absorbed by the passing molecules.
A rod-like carbon dioxide molecule will absorb with slightly different efficiencies depending on how it rotates relative to the light's polarization. Analyzing the oscillating signal allowed the team to observe just how fast and in what direction the molecules were tumbling after hitting the fluid. They found the molecules had a pronounced tendency for a forward, end-over-end "top spin," as if hit by a star Wimbledon tennis player, with the rate of tumbling strongly correlated with how its molecular rotation is aligned relative to the light path.
"To know how this happens at the molecular level—how things bounce, skip, spin, tumble, push and pull—represents a big leap in our understanding," says Nesbitt. "Experiments of this sort help build that understanding."
In addition, Nesbitt says, observing how gas molecules of different shapes twist and rotate after striking a liquid reveals a lot about the nature of the fluid surface—how "rough" it is from the disturbance of microscopic waves and how that roughness affects interactions with gases.
JILA is a research institution operated jointly by NIST and the University of Colorado. The research was supported by the Air Force Office of Scientific Research and the National Science Foundation.
* B.G. Perkins, Jr., and D.J. Nesbitt. Stereodynamics in state-resolve scattering at the gas-liquid interface. Proceedings of the National Academy of Science, Early Edition.
####
About NIST
Founded in 1901, NIST is a non-regulatory federal agency within the U.S. Department of Commerce. NIST's mission is to promote U.S. innovation and industrial competitiveness by advancing measurement science, standards, and technology in ways that enhance economic security and improve our quality of life.
For more information, please click here
Contacts:
Michael Baum
(301) 975-2763
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
![]() Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








