Home > Press > Metals Shape Up with a Little Help from Friends
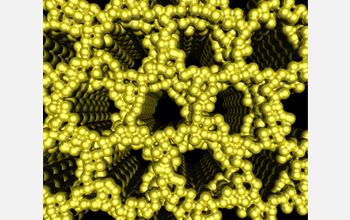 |
| After etching away the carbon material left from the use of intermediary polymers to organize the metal nanoparticles, the platinum structure features large (0.01 micrometers) hexagonal pores. The illustration depicts the completed porous platinum structure. This nanostructured platinum is the product of a radically innovative method for shaping metals developed by Cornell researchers. These porous metal structures have the capability to transform the development of catalysts for fuels cells and materials for microchip fabrication.
Credit: Courtesy of Scott Warren & Uli Wiesner, Cornell University |
Abstract:
New method 'self-assembles' metal atoms into porous nanostructures
Metals Shape Up with a Little Help from Friends
Arlington, VA | Posted on June 28th, 2008For 5,000 years the only way to shape metal has been by the "heat and beat" technique. Even with modern nanotechnology, metalworking involves carving metals with electron beams or etching them with acid.
Now Cornell researchers have developed a method to self-assemble metals into complex configurations with structural details about 100 times smaller than a bacterial cell by guiding metal particles into the desired form using soft polymers.
"I think this is ingenious work that takes the fundamental concepts of polymer science and applies them to make metals in a totally novel way," said Andrew Lovinger, the director of the Polymers Program at the National Science Foundation. "In so doing, it opens the door to all kinds of new possibilities."
Applications include making more efficient and cheaper catalysts for fuel cells and industrial processes, and creating "plasmonic" surface structures capable of carrying more information across microchips than conventional wires do.
"The polymer community has tried to do this for almost 20 years," said Uli Wiesner, Cornell professor of materials science and engineering, who reports on the new method in the June 27, 2008, issue of the journal Science. "But metals have a tendency to cluster into uncontrolled structures."
Wiesner's research team has now developed a method to overcome this globby inclination of metals. First, metal nanoparticles measuring about 2 nanometers (nm) or 10-20 atoms in diameter, are coated with an organic material known as a ligand. The ligands form thin jackets around the metal atoms, changing their surface chemistry. Keeping the ligand jackets thinly tailored is a key factor that permits the volume of metal in the final structure to be large enough to hold its shape when the organic materials are eventually removed.
The jacketed metal atoms are then put in a solution containing block co-polymers, a kind of nano-scaffolding material. The innovative use of the ligands allows for the metal nanoparticles to be dissolved--even at high concentrations--in such a solution. A block co-polymer is made up of two different long chains, or blocks, of molecules linked together to form a predictable pattern. In the experiment, depicted in the illustration at right, ligand-coated platinum nanoparticles (shown as blue and gray balls) are nestled amongst the block co-polymers (shown as blue and green strands).
After the ligand-coated nanoparticles and polymers assemble in regular patterns, the material is heated to high temperatures in the absence of air to convert the polymers to a carbon scaffold. The scaffold is then allowed to cool. Because the metal nanoparticles have a very low melting point, without the carbon scaffold they would stubbornly fuse together in an uncontrolled fashion. Using this process, the carbon scaffold can be etched away with an acid, leaving behind a structured solid metal.
The Cornell group used the new method to create a platinum structure (see illustration above) with uniform hexagonal pores, each on the order of 10 nm across--a much larger diameter than previous attempts have been able to produce. Platinum is, so far, the best available catalyst for fuel cells, and a spacious pore structure allows fuel to flow through and react over a larger surface area.
"It opens a completely novel playground because no one has been able to structure metals in bulk ways using polymers," Wiesner explained. "In principle, if you can do it with one metal you can do it with others or even mixtures of metals."
In addition to making porous materials for catalysis, the researchers said, the technique could be used to create finely structured metals on surfaces, a key to transform the field of plasmonics, which studies the interactions among metal surfaces, light, and density waves of electrons, known as plasmons. Currently, researchers are investigating the use of plasmons to transmit more information across metal wires in microchips and to improve optics applications, like lasers, displays, and lenses.
The research team was led by Uli Wiesner at Cornell University and included Francis DiSalvo, the J.A. Newman Professor of Chemistry and Chemical Biology, and Sol Gruner, the John L. Wetherill Professor of Physics, both at Cornell, and other undergraduate and graduate students.
The research was funded by the National Science Foundation and the Cornell Fuel Cell Institute.
####
About National Science Foundation
The National Science Foundation (NSF) is an independent federal agency that supports fundamental research and education across all fields of science and engineering, with an annual budget of $6.06 billion. NSF funds reach all 50 states through grants to over 1,900 universities and institutions. Each year, NSF receives about 45,000 competitive requests for funding, and makes over 11,500 new funding awards. NSF also awards over $400 million in professional and service contracts yearly.
For more information, please click here
Contacts:
Media Contacts
Bill Steele
Cornell University
(607) 255-7164
Lisa-Joy Zgorski
National Science Foundation
(703) 292-8311
Brandon Michael Garcia
National Science Foundation
(703) 292-8612
Program Contacts
Andrew J. Lovinger
National Science Foundation
(703) 292-4933
Principal Investigators
Uli Wiesner, Cornell University
(607)255-3487
Copyright © National Science Foundation
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Science and Engineering Statistics
Science and Engineering Statistics
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Self Assembly
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
![]() Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








