Home > Press > Towards bio-inspired hydrogen production without noble metals
 |
Abstract:
Using hydrogen as an energy vector and in fuel cells may provide solutions to the specific energy challenges of the 21st century. Hydrogen production is currently based on the catalytic properties of "noble" metals such as platinum. For the first time, researchers at the joint Laboratoire de chimie et biologie des métaux (metal chemistry and biology, CEA-CNRS-Université Joseph Fourier, CEA's Grenoble site) have succeeded in producing hydrogen with a molecular system that doesn't require a noble metal catalyst. This outcome has important implications for the financial future of hydrogen energy and was published on 4 January in the journal Angewandte Chemie International Edition.
Towards bio-inspired hydrogen production without noble metals
France | Posted on January 25th, 2008Research to improve hydrogen production is based largely on chemical reactions observed during photosynthesis in plants. More specifically, certain micro-organisms produce hydrogen from water with the help of light. To reproduce and adapt these processes, researchers have developed molecular systems capable of both photosensitisation, which captures light energy, and catalysis, which uses the energy collected to liberate hydrogen from water. To date, all the technological systems developed to produce or use hydrogen rely on noble metals(1) such as platinum. But platinum reserves are limited. The metal's scarcity and cost are obstacles to the long-term financial prospects of hydrogen technologies, despite efforts to reduce the quantities used in electrolysers and fuel cells. Current research focuses on alternatives to platinum, by developing catalysts based on metals which are naturally more abundant and less expensive, such as those used by natural organisms (iron, nickel, cobalt, manganese).
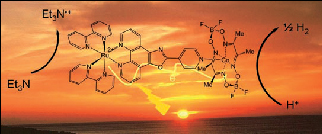
A new system has been developed using a cobalt-based catalyst. Supramolecular in nature, it plays the role of both the photosensitiser and the catalyst. With the help of light, the electrons from the organic molecule are used to liberate hydrogen from water. This is catalysed by cobalt with greater efficiency than comparable systems using noble metals (Pd, Rh and Pt). Ruthenium is still used as the photosensitiser (Ru, left side of the figure); one of the next steps in this work will be finding an alternative.
While the ultimate goal is still to use water as a proton and electron source (to avoid adding an organic molecule), this outcome represents considerable progress towards the photoproduction of hydrogen.
Notes :
1) Historically, noble metals were the precious metals used to make jewellery (gold, silver, platinum). Chemists define them as metals which do not oxidise easily. Today this term is applied to metals present at low levels in the earth's crust, making them both rare and costly (palladium, rhodium, iridium, osmium and ruthenium)
####
For more information, please click here
Contacts:
CEA (French Atomic Energy Commission)
Stéphane Laveissière
+33 (0 1 64 50 27 53
CNRS (French National Centre for Scientific Research)
Claire Le Poulennec
+33 (0) 1 44 96 49 88
Université Joseph Fourier
Muriel Jakobiak
+33 (0) 4 76 51 44 98
Copyright © CNRS
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








