Home > Press > Researchers measure carbon nanotube interaction
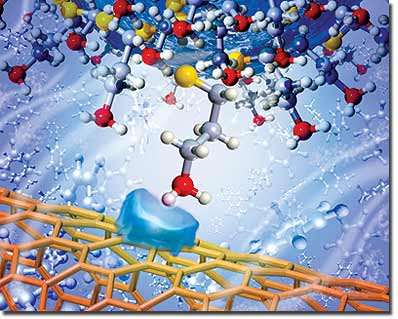 |
| An artist's representation of an amine functional group attached to an AFM tip approaching a carbon nanotube surface in toluene solution. Translucent blue shape on the nanotube represents the polarization charge forming on the nanotube as the result of the interaction with the approaching molecule. Chemical force microscopy measures the tiny forces generated by this single functional group interaction. (Illustration by Scott Dougherty, LLNL) |
Abstract:
Carbon nanotubes have been employed for a variety of uses including composite materials, biosensors, nano-electronic circuits and membranes.
Researchers measure carbon nanotube interaction
LIVERMORE, CA | Posted on October 16th, 2007While they have proven useful for these purposes, no one really knows much about what's going on at the molecular level. For example, how do nanotubes and chemical functional groups interact with each other on the atomic scale? Answering this question could lead to improvements in future nano devices.
In a quest to find the answer, researchers for the first time have been able to measure a specific interaction for a single functional group with carbon nanotubes using chemical force microscopy - a nanoscale technique that measures interaction forces using tiny spring-like sensors. Functional groups are the smallest specific group of atoms within a molecule that determine the characteristic chemical reactions of that molecule.
A recent report by a team of Lawrence Livermore National Laboratory researchers and colleagues found that the interaction strength does not follow conventional trends of increasing polarity or repelling water. Instead, it depends on the intricate electronic interactions between the nanotube and the functional group.
"This work pushes chemical force microscopy into a new territory," said Aleksandr Noy, lead author of the paper that appears in the Oct. 14 online issue of the journal, Nature Nanotechnology.
Understanding the interactions between carbon nanotubes (CNTs) and individual chemical functional groups is necessary for the engineering of future generations of sensors and nano devices that will rely on single-molecule coupling between components. Carbon nanotubes are extremely small, which makes it particularly difficult to measure the adhesion force of an individual molecule at the carbon nanotube surface. In the past, researchers had to rely on modeling, indirect measurements and large microscale tests.
But the Livermore team went a step further and smaller to get a more exact measurement. The scientists were able to achieve a true single function group interaction by reducing the probe-nanotube contact area to about 1.3 nanometers (one million nanometers equals one millimeter).
Adhesion force graphs showed that the interaction forces vary significantly from one functionality to the next. To understand these measurements, researchers collaborated with a team of computational chemists who performed ab initio simulations of the interactions of functional groups with the sidewall of a zig-zag carbon nanotube. Calculations showed that there was a strong dependence of the interaction strength on the electronic structure of the interacting molecule/CNT system. To the researchers delight, the calculated interaction forces provided an exact match to the experimental results.
"This is the first time we were able to make a direct comparison between an experimental measurement of an interaction and an ab initio calculation for a real-world materials system," Noy said. "In the past, there has always been a gap between what we could measure in an experiment and what the computational methods could do. It is exciting to be able to bridge that gap."
This research opens up a new capability for nanoscale materials science. The ability to measure interactions on a single functional group level could eliminate much of the guess work that goes into the design of new nanocomposite materials, nanosensors, or molecular assemblies, which in turn could help in building better and stronger materials, and more sensitive devices and sensors in the future.
Other Livermore researchers include, Raymond Friddle, Melburne LeMieux and Alexander Artyukhin.
####
About Lawrence Livermore National Laboratory
Founded in 1952, Lawrence Livermore National Laboratory is a national security laboratory, with a mission to ensure national security and apply science and technology to the important issues of our time. Lawrence Livermore National Laboratory is managed by Lawrence Livermore National Security, LLC for the U.S. Department of Energy's National Nuclear Security Administration.
For more information, please click here
Contacts:
Anne M.Stark
Phone:(925)422-9799
Copyright © Lawrence Livermore National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Molecular Nanotechnology
![]() Scientists push the boundaries of manipulating light at the submicroscopic level March 3rd, 2023
Scientists push the boundaries of manipulating light at the submicroscopic level March 3rd, 2023
![]() First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
![]() Nanotech scientists create world's smallest origami bird March 17th, 2021
Nanotech scientists create world's smallest origami bird March 17th, 2021
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Sensors
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








