Home > Press > The smallest piece of ice reveals its true nature
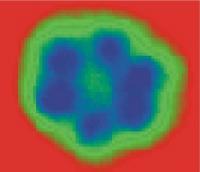 |
| The "smallest particle of ice " -- a water hexamer as seen by STM (about 1 nanometer wide, left) and quantum mechanics (right).
Credit: London Centre for Nanotechnology, 2007 |
Abstract:
Collaborative research between scientists in the UK and Germany (published in this week's Nature Materials) has led to a breakthrough in the understanding of the formation of ice. Dr Angelos Michaelides of the London Centre for Nanotechnology (formerly of the Fritz-Haber Institut der Max-Planck Gesellschaft in Berlin) and Professor Karina Morgenstern of the Leibniz University Hannover have combined experimental observations with theoretical modelling to reveal with unprecedented resolution the structures of the smallest pieces of ice that form on hydrophobic metal surfaces.
The smallest piece of ice reveals its true nature
London, UK | Posted on June 21st, 2007The results provide information about the process of ice nucleation at a molecular level and take science a significant step closer to understanding the mysterious process through which ice forms around microscopic dust particles in the upper atmosphere. Because this is the basis of cloud formation, knowing how different particles promote ice formation is crucial for climate change models.
The authors began by cooling down a metallic surface to 5 degrees above absolute zero (around -268 Celsius) at which temperature it was possible to "trap" and obtain images of the smallest possible pieces (hexamers) of ice using a scanning tunnelling microscope (STM). The hexamer - the simplest and most basic "snow flake" - is composed of just six water molecules. Other ice nanoclusters containing seven, eight and nine molecules were also imaged.
On the difficulties of imaging these ice clusters, Prof Morgenstern said: "Scientists have long struggled to resolve single water molecules within ice clusters, because they are so vulnerable to damage induced by electrons - the very thing that creates the image. The high resolution could only be achieved by reducing the current to the smallest value technically possible."
As well as performing experiments, the team used highly-accurate (‘first principles') theoretical models to analyse how such a structure could form. Here the theory provided some surprising insights. In ice, water molecules usually bond to each other with equal strength but with the ice nanoclusters the team identified a pattern of alternating shorter and longer bonds between the water molecules. This pattern provided new information about the ability of water molecules to share their hydrogen bonds, revealing a hitherto unknown competition between the ability of water molecules to bind to a metal surface and simultaneously accept hydrogen bonds.
Dr Michaelides said, "We are all familiar with the freezing of water. It features prominently in our daily lives, from fridge freezers to winter snow. Despite all this, the question of how individual water molecules come together and give birth to ice crystals remains mysterious. Our research provides an insight into the most important and ubiquitous type of ice nucleation event, namely heterogeneous nucleation. State-of-the-art experimental and theoretical techniques allowed us to "watch" and accurately model what happens at very low temperatures."
The research makes it possible to explain the ways in which water structures form on different substrates, such as transition metals and salt surfaces. It may also provide a new way of thinking about the structure of ice clusters that form on solid surfaces in general, opening the door for applications in a variety of fields as diverse as astronomy, electrochemistry, and energy research. It also takes us a step closer to understanding how water interacts with different aerosols and dust particles in the atmosphere, processes which drive cloud formation and have a large impact on the planet's climate.
####
About University College London
Founded in 1826, UCL was the first English university established after Oxford and Cambridge, the first to admit students regardless of race, class, religion or gender, and the first to provide systematic teaching of law, architecture and medicine. In the government’s most recent Research Assessment Exercise, 59 UCL departments achieved top ratings of 5* and 5, indicating research quality of international excellence.
UCL is the fourth-ranked UK university in the 2006 league table of the top 500 world universities produced by the Shanghai Jiao Tong University. UCL alumni include Mahatma Gandhi (Laws 1889, Indian political and spiritual leader); Jonathan Dimbleby (Philosophy 1969, writer and television presenter); Junichiro Koizumi (Economics 1969, Prime Minister of Japan); Lord Woolf (Laws 1954, Lord Chief Justice of England & Wales); Alexander Graham Bell (Phonetics 1860s, inventor of the telephone), and members of the band Coldplay.
About the London Centre for Nanotechnology
The London Centre for Nanotechnology is a joint enterprise between University College London and Imperial College London. In bringing together world-class infrastructure and leading nanotechnology research activities, the Centre aims to attain the critical mass to compete with the best facilities abroad. Furthermore by acting as a bridge between the biomedical, physical, chemical and engineering sciences the Centre will cross the 'chip-to-cell interface' - an essential step if the UK is to remain internationally competitive in biotechnology. Website: http://www.london-nano.com
Funding:
Work at the Fritz-Haber-Institut der Max-Planck-Gsesllschaft was funded by the Deutschen Forschungsgemeinschaft (DFG) and European Science Foundation through a European Young Investigator Award (EURYI). See http://www.esf.org/euryi
Work at the London Centre for Nanotechnology was funded by the Engineering and Physical Sciences Research Council (EPSRC) and European Science Foundation through a European Young Investigator Award (EURYI). See http://www.esf.org/euryi
Work at the Leibniz University of Hannover was funded by the Deutsche Forschungsgemeinschaft (DFG).
For more information, please click here
Contacts:
Dave Weston
44-020-767-97678
mobile: +44 (0) 7733 307 596
out of hours +44 (0)7917 271 364
Copyright © University College London
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








