Home > Nanotechnology Columns > ONAMI > DC Report February 2007: The Two Big Nano Action Items, or Why Commercialization and EHS Go Together Like Peanut Butter and Chocolate
 |
Skip Rung President and Executive Director ONAMI |
Abstract:
The perennial two big discussion topics on nanotechnology in policy circles are commercialization and EHS risk assessment. There are compelling reasons why these should be thought of and addressed in both policy and investment simultaneously. The "green nanotechnology" approach is analogous to concurrent development as practiced by high tech industry (or to the felicitous merger of peanut butter and chocolate as practiced by confectioners).
February 5th, 2007
DC Report February 2007: The Two Big Nano Action Items, or Why Commercialization and EHS Go Together Like Peanut Butter and Chocolate
A nanotube-based RAM cell that greatly increases the density of embedded non-volatile memory while decreasing related capital cost and process complexity (and chemical consumption in proportion). A bio-nano chip that enables personalized diagnosis and treatment plan development that literally means the difference between life and death for patients with genetic disorders. A way to tune engineered polymers for greatly enhanced performance and reliability in a myriad of applications. A transparent organic conducting film that promises to replace a rare, toxic, and brittle metal compound. An electrode material that eliminates toxic mercury and triples the lifetime of hearing aid batteries.
More of the usual overhyped promises by nano-enthusiasts, right? Wrong. These are snapshots from a jam-packed briefing, organized by the NanoBusiness Alliance, by fast-growing nanotechnology company CEOs and CTOs for examiners at the U.S. Patent and Trademark Office. All of these products have been prototyped and are either shipping or will be very soon.
All of these exciting developments (and many more which could be mentioned) have three things in common:
First, they offer stunning performance benefits to existing product categories (e.g. semiconductors, medical diagnostics, consumer displays);
Second, they deliver environmental benefit by direct or indirect saving of material/reduction of solid waste and/or substitution of safer materials for toxic metals;
and
Third, all but one (the nano bio chip) involves the use of nanoparticles or nanotubes/wires as intermediates in the production of a product in which the nanomaterial will be integrally bound.
Consumer/environmental exposure to disaggregated nanomaterial in these cases is very unlikely, but factory worker exposure is a risk that is being proactively managed. In a growing number of cases, nanotechnology companies like these are cooperating with the EPA voluntary material testing program and inviting NIOSH into their facilities to assess worker exposure risks and the appropriateness of protection measures. According to the NanoBusiness Alliance, this same level of proactivity and trust between businesses and regulatory bodies does not exist in Europe. Over here, I interpret this to mean that our nano entrepreneurs are confident in their success, including their ability to run safe factories and ship safe products, but also eager to engage the best technical resources to ensure safety as they increase volumes, improve productivity and reduce manufacturing cost.
As in past years, the NanoBusiness delegates (including your humble columnist) met with key members (i.e. Science, Environmental Protection, Commerce, Finance, Appropriations) of the US Senate and House of Representatives to discuss the importance of research and innovation and how best to ensure the social and economic returns that everyone wants to see.
This year, we think the message got through that technology entrepreneurship and early stage investment is both crucial - and endangered, i.e. Sarbanes Oxley and other measures intended to combat corporate crime and protect small investors have ironically harmed early stage investing and domestic IPOs/registrations (the data here seems clear), have been absorbed without significant pain by big business, and have created riches that Croesus would envy for $1000/hour partners at large accounting firms awash in lucrative new auditing work. There are hopeful signs that targeted improvements in the climate for investors in early stage nano companies will improve, e.g. relaxation of the most burdensome (and unnecessary) bureaucracy, and tax credits for qualified equity investments involving research commercialization.
But the really big issues and discussions were the two perennial ones:
1. How do we capitalize on the large federal research investment to create products, companies and jobs - not just publications, citations and patents,
and
2. How do we ensure that nanotechnology (i.e. engineered nanomaterials) will be safe for workers, consumers and the environment?
My "insight" this time was that these two big "nano action items" should not be pursued in opposition, should not be pursued in isolation, but should rather be pursued in close collaboration (e.g. like chocolate and peanut butter to make Reese's Peanut Butter Cups), since they are really about the same thing - the wellbeing of American citizens - both economically (high-wage jobs enabled by innovation) and socially (personal health, living environment).
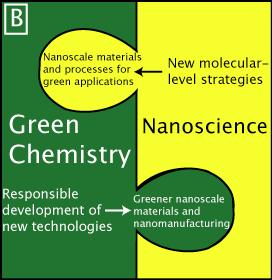 |
(Image credit: McKenzie and Hutchison, Chemistry Today 2004)
We will get good results much faster, and with far less confusion and inefficiency, if we treat this as a case of "concurrent development". We won't measure and test for the right and relevant EHS parameters unless we do so with materials that the market wants (otherwise little volume and little risk) produced by realistic manufacturing processes (otherwise material purity and match of characteristics to what society will experience are in doubt). Similarly, the risk profile seen by investors and company management will be much reduced by participating in widely agreed upon synthesis techniques, factory protocols and packaging/shipping/handling practices that are designed to be correct from the start.
As with other cases of concurrent development, this will be a collaborative and learning process every step of the way, but successful technology companies know that this approach is much better and faster than a serial "over the wall" (Central R&D to Development Lab to Production Department to Quality Assurance) methodology that no serious competitor would think of using today. US industry nearly lost everything due to its late embrace of total quality/ kaizen, lean manufacturing and other advanced management practices. Let's not re-learn that painful lesson with nanotechnology.
This topic is a major thrust for ONAMI, and we'll be talking about it at length at two upcoming events - ONAMI Safer Nano 2007 (see http://www.greennano.org/marchevent.html ) March 12-13, and the 2007 Micro Nano Breakthrough Conference (see http://www.micronbc.org ) September 10-12.
I hope you'll join us.
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








